2018
Iron Self-diffusion in Fe5Ge3 thin film
Iron-germanides are widely investigated due to their possible application in nanoelectronics and spintronics applications. The feasibility of these materials in industrial application is highly dependent on their structural stability against temperature, therefore it is indispensable to understand temperature induced diffusion processes in this system. [57Fe5Ge3(36 Å)/nFe5Ge3(62 Å)]10 isotope-periodic multilayer has been prepared by molecular beam epitaxy, in order to study iron self-diffusion in Fe5Ge3. By using neutron reflectivity technique, which is sensitive to atomic scale diffusion lengths, we have determined the pre-exponent factor and activation energy as D0 = (8.22 ± 3.8) × 10-18 m2s−1 and Ea = (0.28 ± 0.02) eV respectively.
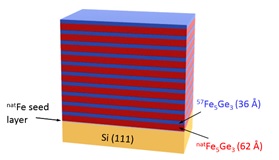
Figure 1. Layer structure of Fe5Ge3 isotope periodic multilayer structure.
The effect of carboxylic acids on the core/shell structure of iron oxide nanoparticles
Core/shell nanoparticles have been in the center of scientific interest due to their possible applications in medicine and catalysis. The formation of magnetite/maghemite core/shell structure was studied by Mössbauer-, Raman- and infrared spectroscopies as well as by electronmicroscopy and x-ray diffractometry in coprecipitated iron oxide based nanocomposites functionalized with various carboxylic acids. The core/shell ratio of the nanomagnetites can be changed by the oxidation or the reduction of the particles. It was found that sometimes the phases cannot be determined with Mössbauer spectroscopy at room- or liquid nitrogen temperatures, but only if the samples are
measured at the temperature of liquid helium. Our results have shown that alongside with the preparation atmosphere the time of the washing is also crucial parameter during the synthesis of nanomagnetites. We have also found that the previously found correlation between that carboxylic acids and core/shell ratio of the nanomagnetites, can be caused by the different acidity of the carboxylic acids used for the functionalization of the iron oxide nanoparticles. These novel finding can be helpful to adjust the core/shell ratio of the coprecipitated carboxylic acid coated nanomagnetites.
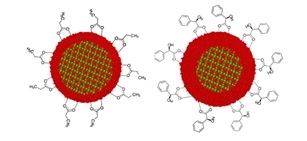
Figure 2. Schematic illustration of coating with carboxylic acids on nanomagnetite with the change of the thickness of meghemite shell.



